Abbott Covid Test Positive Control
The test does not need any additional. The presence of the test line T and the control line C within the result window regardless of which line appears first indicates a positive result.

Rapid Covid Tests What You Should Know About Accuracy
5 Hold the buffer bottle vertically and fill the extraction tube.
Abbott covid test positive control. Abbott BinaxNOWTM COVID-19 Ag Card Test Helpful Testing Tips continued Before the Test. COVIDPC - Positive Control Template with an expected threshold cycle Ct value range serves as a. For more information on ID NOW check out this article.
Only the swabs provided with the kit are approved for use with test. Each test kit comes with a positive and negative control swab to ensure the test is working properly. Collected swabs were transferred into dedicated sample collection tubes containing a sampling buffer and transported to the laboratory.
Panbio is a highly effective and safe test. The importance of conducting negative and positive control. The negative control swab ensures appropriate negative results are obtained.
BinaxNOW COVID-19 Ag Control Swab Kit 10 Positive Swabs 190-010. To test for this a positive control is used. This is standard for most rapid diagnostic tests throughout the world.
The presence of only the control line C and no test line T within the result window indicates a negative result. Abbott RealTime SARS-CoV-2 Positive Control 8 vials 13 mL per vial Contains non-infectious recombinant Sindbis virus containing SARS-CoV-2 RNA sequences 10 ammonium sulfate and 79. Negative results from antibody testing do not rule out SARS-CoV-2 infections particularly.
Not approved for sale in the USA. Store kit at 2-30C 356-86 F. The testing kits in question were made by Abbott and were called Panbio COVID-19 Ag Rapid Test Device which is an antigen test.
BinaxNOW COVID-19 Ag Card 40 Tests 195-080. If stored in a refrigerator allow time to warm up to room temperature. Sterile swabs foam for use with the ID NOW COVID-19 Test.
The tests can be used in point-of-care settings and at home with an online service provided by eMed. This test is used on our ID NOW instrument. In the documentation for its Panbio COVID-19 antigen rapid test kit for example Abbott also lists the negative and positive control swabs and specifies they are not to be used for specimen.
41FK11 CE CONTENTS. Abbotts BinaxNOW COVID-19 Ag Card test can identify these antigens which are typically detected after symptoms start. 3 Remove the test device from the foil pouch placing it on a flat horizontal and clean surface.
Our rapid antigen test BinaxNOW COVID-19 Ag Card provides results in 15 minutes when used to test individuals suspected of COVID-19. Positive individuals should be isolated per Ohio Department of Health guidance. Greg Abbott was one of the more than 20000 Texans to test positive for coronavirus Tuesday his office said in a statement as cases and hospitalizations in.
Results from the simple nasal swab are available in 15 minutes through testing individuals suspected of COVID-19. COVID-19 Ag Card kits contain a Positive Control Swab and Sterile Swabs th at can be used as a Negative Control Swab. Illinois-based Abbott Laboratories has just been.
Assay controls should be tested concurrently with all test samples in each instrumental run. The Panbio COVID-19 Ag rapid test device by Abbott Lake Country IL USA is a membrane-based immunochromatography assay which detects the nucleocapsid protein of SARS-CoV-2 in nasopharyngeal samples. Test kit reagentscards must be at room temperature before use.
This is a swab that has been treated in such a way that it should give a positive result when run through the test so that health workers know they. Abotts pocket-sized 5 15-minute COVID-19 test was just granted emergency use authorization. Do not freeze kits.
Presently a positive test result from a POC serological test for SARS -CoV-2 COVID-19 shows that an individual has antibodies that likely resulted from an infection with SARS -CoV-2 or possibly a related coronavirus. 4 Label test device with positive or negative control. 25 Test Devices 1 Buffer 9 mLbottle 25 Extraction Tubes 25 Extraction Tube Caps 1 Positive Control Swab.
COVID-19 Ag Rapid Test Quality Control Log Sheet 2 Place tube rack on the level work space to be utilized f or testing. The positive control swab ensures sample elutionlysis and workflow were performed correctly. These control swabs are not used as part of patient testing.
Our rapid molecular point-of-care test detects COVID-19 in 13 minutes or less. Alesia Scott spokesperson for Abbott told AFP. A positive test result when the person is symptomatic or has been exposed to COVID-19 indicates that SARS-CoV-2 antigen was detected and that the individual is very likely infected and considered a COVID-19 case.
WWWPOC-COVIDABBOTT ORDER INFORMATION PANBIO COVID-19 Ag RAPID TEST DEVICE NASAL Product not available in all countries. These swabs will monitor the entire assay. Optional COVID-19 Swab Transport Tube Accessory Pack 24 Tubes Technical Support Line.

Controls Of The Panbio Tm Covid 19 Ag Test Abott A Supplied Pcs 2 Download Scientific Diagram

Abbott On Twitter We Have A Positive And Negative Control Swab In Each Test Kit To Ensure The Test Is Working Properly This Is Standard For Most Rapid Diagnostic Tests Throughout The

Abbott On Twitter The Symbol Means That The Product Contains A Biological Component And Is Required For Regulatory Purposes It Does Not Mean That The Panbio Positive Control Swab Contains Contagious Or

Controls Of The Panbio Tm Covid 19 Ag Test Abott A Supplied Download Scientific Diagram

Quebec Expanding Rapid Covid Tests But Experts Warn Of Limited Use Ctv News
Covid 19 Rapid Antigen Test Wikipedia

Panbio Covid 19 Antigen Rapid Test

Millions More Virus Rapid Tests But Are Results Reported Opb

Andrew Villegas Colorado Public Radio

European Commission On Twitter Today Is The International Day Of Epidemic Preparedness We Are Taking Forward The Lessons Learnt This Year And Building A Stronger European Healthunion Where We Work With
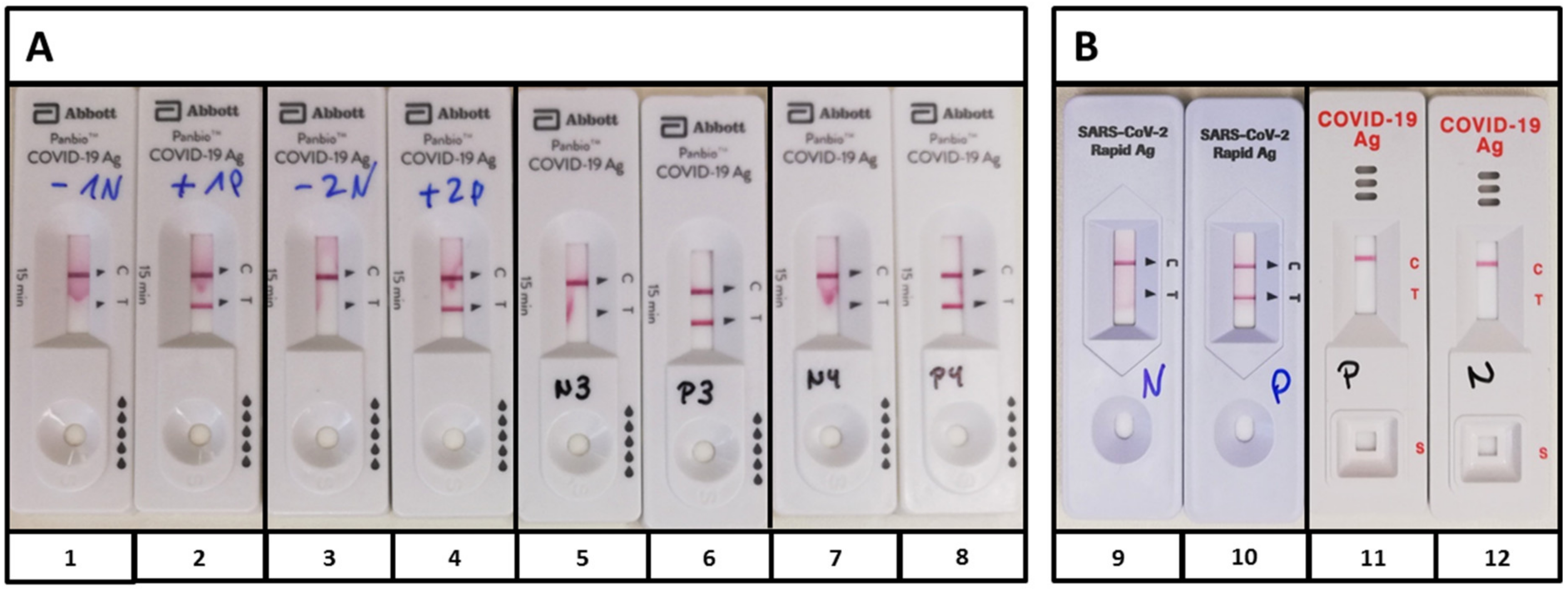
Pathogens Free Full Text Limits And Opportunities Of Sars Cov 2 Antigen Rapid Tests An Experienced Based Perspective Html
Https Extranet Who Int Pqweb Sites Default Files Documents Eul 0587 032 00 Panbio Agrapidtestdevice Nasal V3 Pdf

Binaxnow Covid 19 Antigen Self Test Kit Walgreens

Covid 19 Rapid Test By Abbott Labs Given Emergency Fda Approval Claims Accurate Results In 15 Minutes Abc7 Chicago

How To A Guide For The Binaxnow Covid 19 Self Test Youtube

Abbott Launches Rapid Portable Antigen Test Increasing Covid 19 Testing Capacity In France 2020 09 18 Bioworld

Rapid Antigen Test Is Far Less Sensitive Than Lab Standard Newsroom

