Moderna Vaccine Ingredients Cdc / Allergic Reactions Related To Covid 19 Vaccinations In Allergic Patients American Academy Of Otolaryngology Head And Neck Surgery Aao Hns
The Moderna COVID-19 Vaccine includes the following ingredients. Messenger ribonucleic acid mRNA lipids SM-102 polyethylene glycol PEG 2000 dimyristoyl glycerol DMG cholesterol and 12.
Https Www Mass Gov Doc Covid 19 Vaccine Training The Moderna Supplement Download
CDCs vaccine excipient summary and the National Institutes of Health DailyMed database can also be used as a resource.

Moderna vaccine ingredients cdc. The Janssen vaccine is a recombinant replication-incompetent. PEG is an ingredient in the mRNA vaccines and polysorbate is an ingredient in the JJJanssen vaccine. Moderna COVID-19 Vaccine Vaccine Preparation and Administration Summary General Information Vacc.
PEG and polysorbate are closely related to each other. CDC and FDA are reviewing data involving six reported US. 7 Zeilen Vaccine Ingredients.
What are the ingredients in the moderna covid-19 vaccine. 18 years of age and older. Lipids The Moderna vaccine also requires lipids to help deliver the mRNA to the cells.
Do NOT mix with a diluent. The full list of ingredients for the Moderna vaccine is. If you are allergic to PEG you should not get an mRNA COVID-19 vaccine.
MRNA Like the Pfizer BioNTech vaccine Modernas also uses mRNA technology to build antibodies against COVID-19. Messenger ribonucleic acid mRNA lipids SM-102 polyethylene glycol. COVID-19 vaccination providers must document vaccine administration in their medical record systems within 24 hours of administration and use their best efforts to report.
If You Are Allergic to Polyethylene Glycol PEG or Polysorbate. Aluminum is not an ingredient in the Pfizer-BioNTech and Moderna COVID-19 vaccines as. 2 shots 28 days apart Approved for use in people aged 18 years and older Ingredients.
Maximum of 15 doses per vial Dosage. Moderna Vaccine 94 efective Number of shots. Clipboard list check icon.
Polyethylene glycol PEG 2000 dimyristoyl glycerol DMG 12-distearoyl-sn. Messenger ribonucleic acid mRNA lipids SM-102 polyethylene glycol PEG 2000 dimyristoyl glycerol DMG cholesterol and 12-distearoyl-sn-glycero-3-phosphocholine DSPC. Description Pfizer-BioNTech mRNA Moderna mRNA Janssen viral vector Active ingredient Nucleoside-modified.
Schedule 2-dose series separated by 1 month 28 days. Find a suite of information and materials that are needed for each specific COVID-19 vaccine that cover administration storage and handling safety and reporting. The Moderna COVID-19 Vaccine contains the following ingredients.
The Moderna COVID-19 Vaccine is made of the following ingredients. 4-hydroxybutylazanediylbishexane-61-diylbis2-hexyldecanoate SM-102 Proprietary to Moderna Salts sugars buffers Potassium chloride Tromethamine Monobasic potassium phosphate Tromethamine hydrochloride Sodium chloride Acetic acid. Administer 05 mL Moderna COVID-19 Vaccine by intramuscular IM injection.
The Pfizer-BioNTech and Moderna vaccines are lipid nanoparticle-formulated nucleoside-modified mRNA vaccines encoding the prefusion spike glycoprotein of SARS-CoV-2 the virus that causes COVID-19. Messenger ribonucleic acid mRNA lipids SM-102 polyethylene glycol PEG 2000 dimyristoyl glycerol DMG cholesterol and 12-distearoyl-sn-glycero-3-phosphocholine DSPC. As of April 12 more than 68 million doses of the Johnson Johnson Janssen vaccine have been administered in the US.
Janssen COVID-19 vaccine in persons aged 18 years. Ingredients included in mRNA COVID-19 vaccines As reported in the prescribing information Descriptio n Pfizer-BioNTech COVID-19 vaccine Moderna COVID-19 vaccine mRNA nucleoside-modified mRNA encoding the viral spike S glycoprotein of SARS-CoV-2 nucleoside-modified mRNA encoding the viral spike S glycoprotein of SARS-CoV-2 Lipids 2polyethylene glycol-2000. MRNA Nucleoside-modified mRNA encoding the viral spike S glycoprotein of SARS-CoV-2.
The CDC lists various vaccines that contain aluminum here. Type of Ingredient Examples Purpose Most common source found. Moderna COVID-19 vaccine in persons aged 18 years.
ACIP recommendations and CDC guidance for COVID-19 vaccination ACIP considered anaphylaxis risk during deliberations on Pfizer-BioNTech COVID-19 vaccine during Dec 11-12 2020 meetings Issued interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine CDC issued. Preparing for the Potential. Información acerca de la vacuna contra el COVID-19 de Moderna incluidos el nombre fabricante tipo de vacuna cantidad de dosis cómo se administra y enlaces a información de los ingredientes.
The Moderna COVID-19 Vaccine contains the following ingredients.
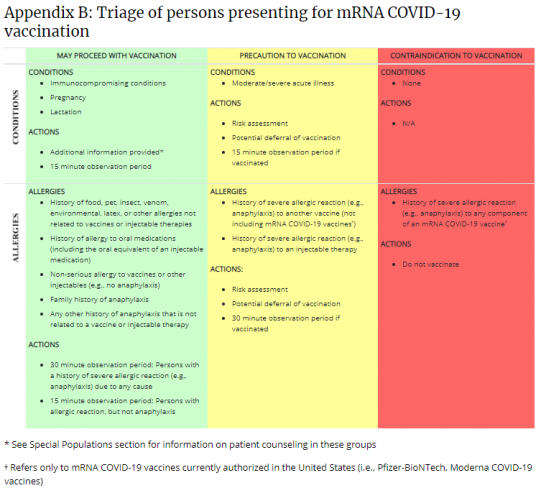
Allergic Reactions Related To Covid 19 Vaccinations In Allergic Patients American Academy Of Otolaryngology Head And Neck Surgery Aao Hns

Okc County Health Department Covid Vaccine
Cdc A New Cdc Study Finds That Mrna Covid 19 Vaccines Are Highly Effective In Preventing Covid 19 Among Health Care And Other Essential Workers Groups More Likely To Be Exposed To The

Allergy And Immunology Patients Coronavirus Guidance

Covid 19 Vaccines Educational Materials For Patients And Healthcare Professionals
Https Www Vcuhealth Org Media Media Featurednewsimages Covid 19 Vaccine Infographic V5 Accessible And With Extra Bullet Ashx

What S In The Pfizer And Moderna Covid Vaccines
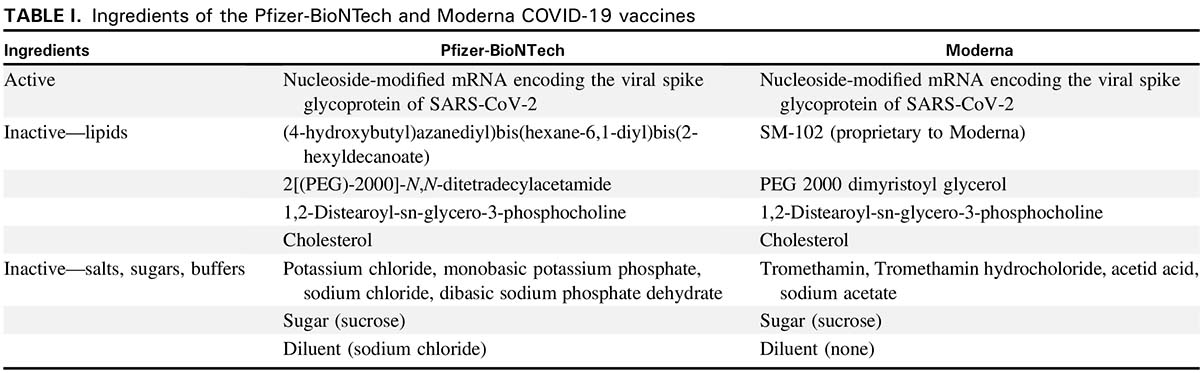
Covid 19 Vaccine Reported Allergic Reactions Allergy Asthma Network
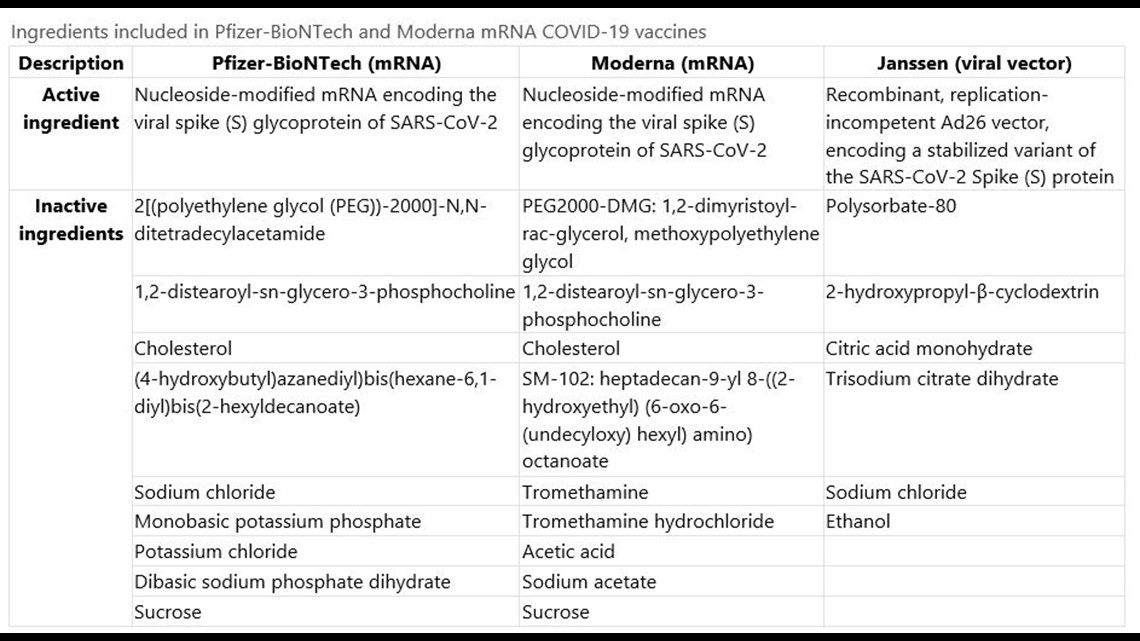
What Is In The Covid 19 Vaccines Wusa9 Com

Pfizer And Moderna Covid 19 Vaccine Ingredients
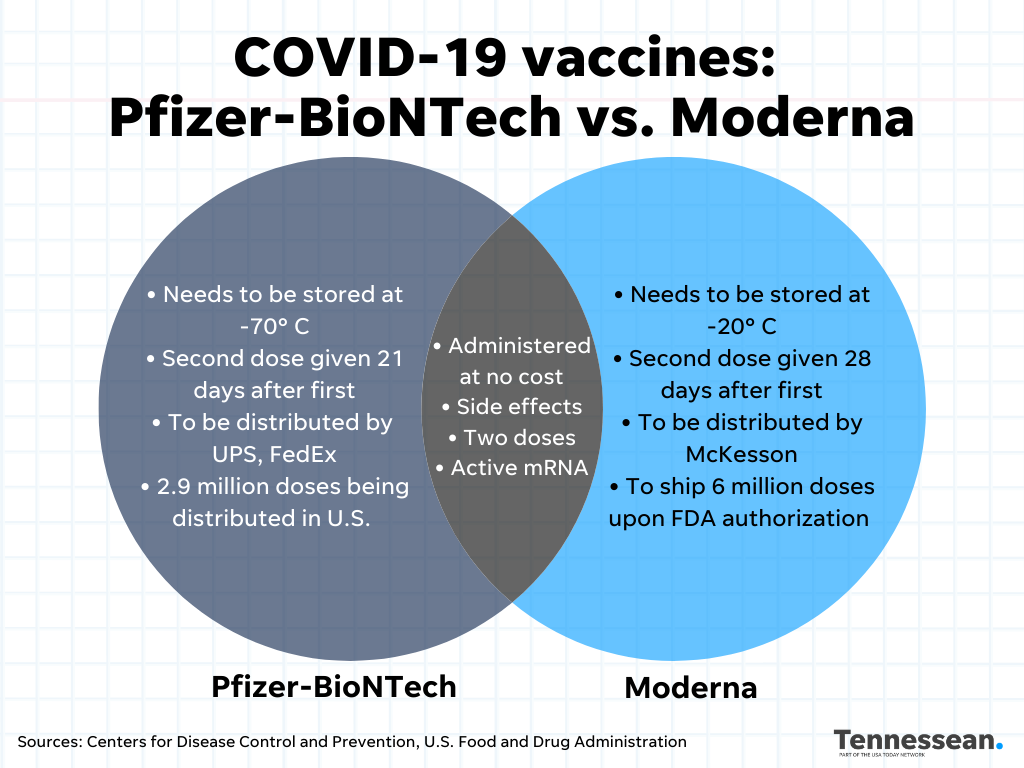
Comparing Pfizer Moderna Covid 19 Vaccine Side Effects Ingredients

What S In The Pfizer And Moderna Covid Vaccines
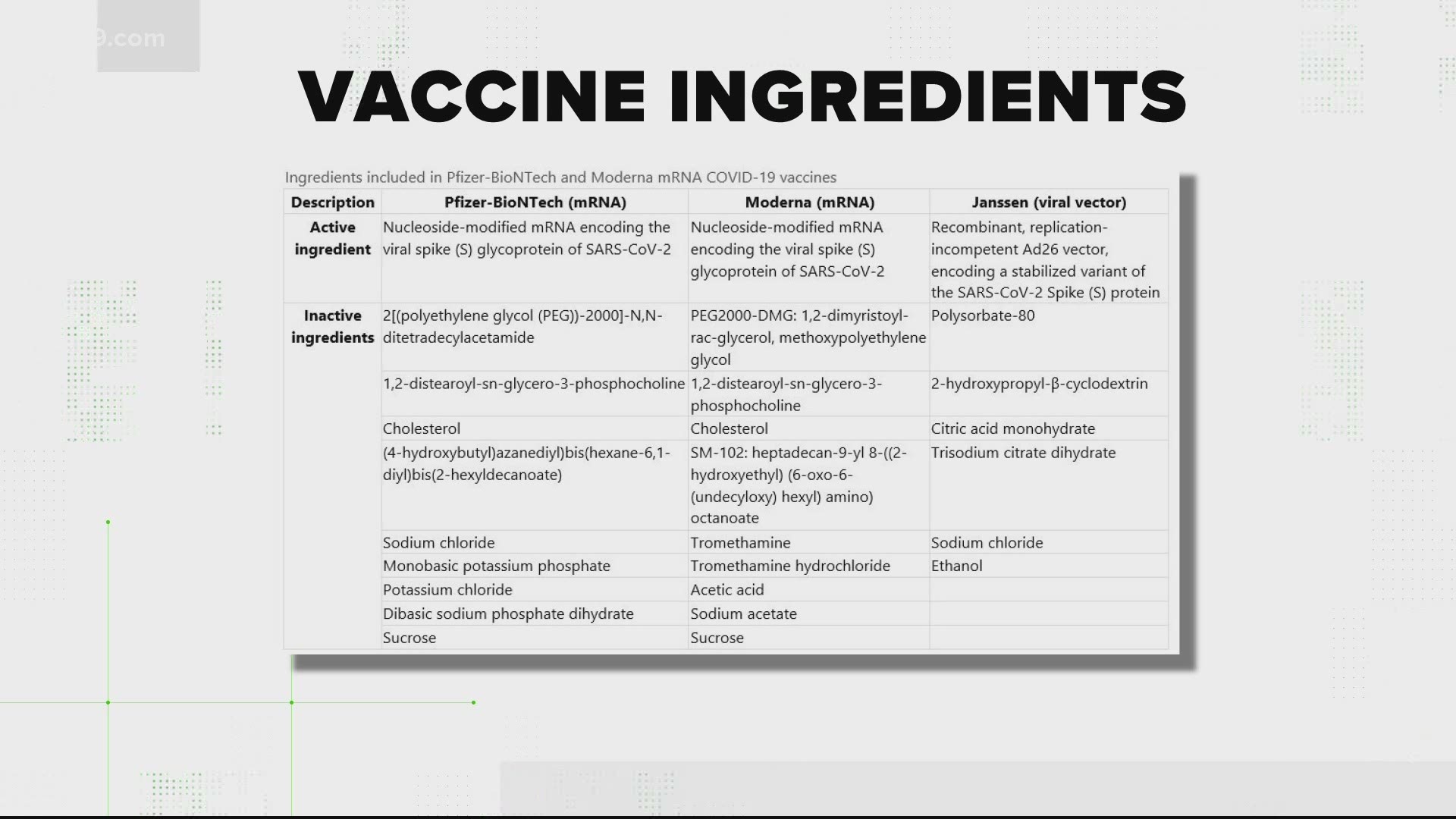
What Is In The Covid 19 Vaccines Wusa9 Com
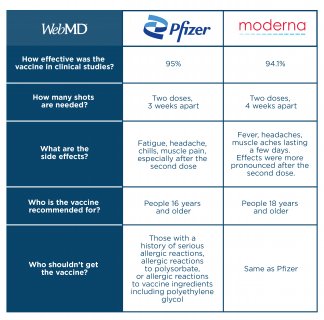
Vaccine Information For Older Adults Senior Lifestyle
Covid 19 Vaccine Allergy Information St Mary S County Health Department




